Cardiovascular diseases such as cardiac arrhythmias (e.g., ventricular fibrillation), contribute to a significant proportion of deaths. Fibrillation produces chaotic excitation waves in the heart muscle, whose biophysical and dynamical origin is a topic of current research aiming at novel therapies, like Low-Energy Anti-fibrillation Pacing (LEAP) or ablation methods. Measuring the spatio-temporal wave pattern is key for understanding their features and developing new control methods. Electrical waves and calcium concentration on the heart surface can be measured optically in a Langendorff perfusion setup with fluorescent dyes, although the image quality is still poor and only activity of the surface can be monitored. Accordingly, R5 based at MPI will develop new methods based on optoacoustic imaging, and machine learning for optogenetic experiments, which can provide new perspectives for optical (using light) arrhythmia control.


Topics
BE-LIGHT brings together an interdisciplinary consortium of 7 top academic institutions, 3 internationally recognized hospitals and an ecosystem of 7 private companies with complementary know-how in photonics, microscopy, artificial intelligence, medical instruments and clinical research, to promote inter-sectoral synergies.
BE-LIGHT’s multi-skill training program will provide the researchers with the complete set of skills that are nowadays key for their success: a broad understanding of how state-of-the-art light-based technologies work, a solid knowledge of machine learning and data processing, and experience of clinical and commercialization processes.
The BE-LIGHT research programme includes 11 application-oriented projects that are structured in 3 work packages: eye diseases and vision (WP1), cardiovascular diseases (WP2) and advanced microscopy for the analysis of cells and tissues involved in the development of diseases (WP3).
An aging population has high risk of ocular disorders (blindness affects 45 million people worldwide). A promising strategy to restore vision is to make specific types of cells in the retina light sensitive by expressing optogenetic proteins that can activate neurons upon light stimulation.
For this, it is necessary to understand how the retina performs complex computations on the visual scene before sending the result to the brain. The goal of R1 based at SU is to develop quantitative models of the function of the retina using a novel optical tool to stimulate individual retinal cells, allowing the differentiation between healthy and degenerated retinas, and other downstream regions.
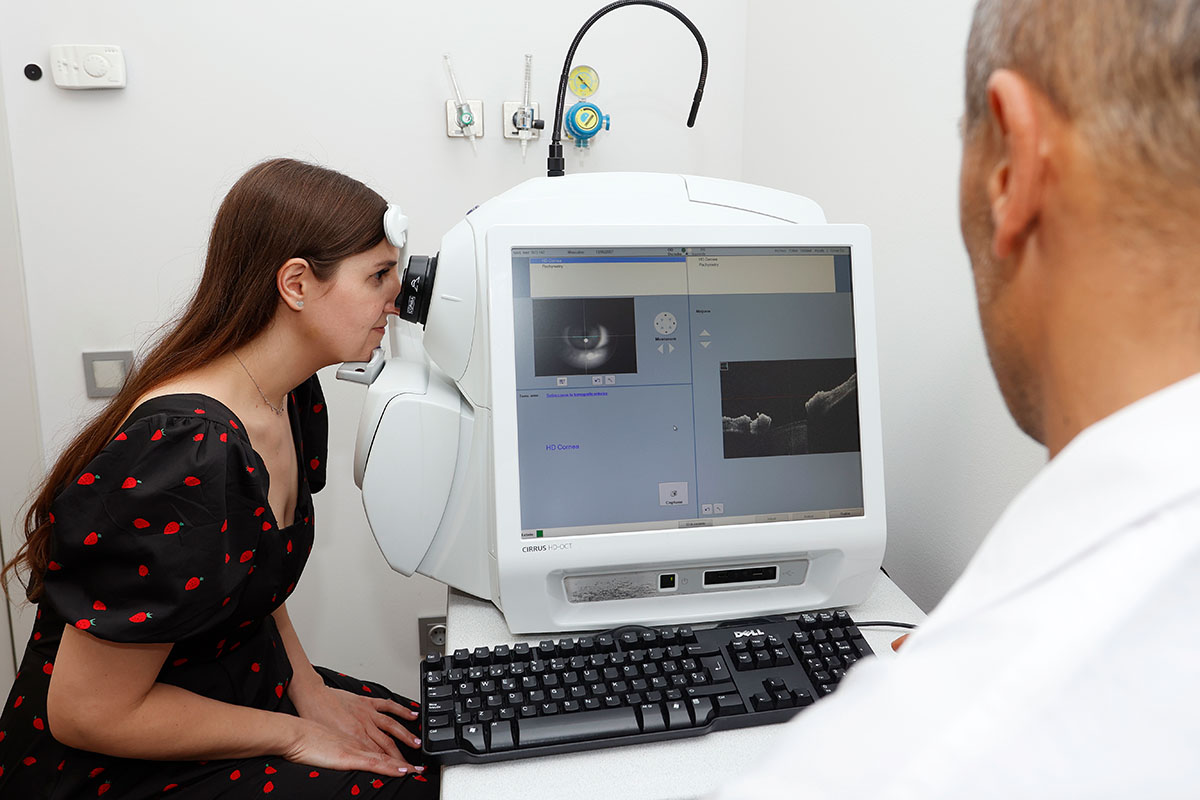
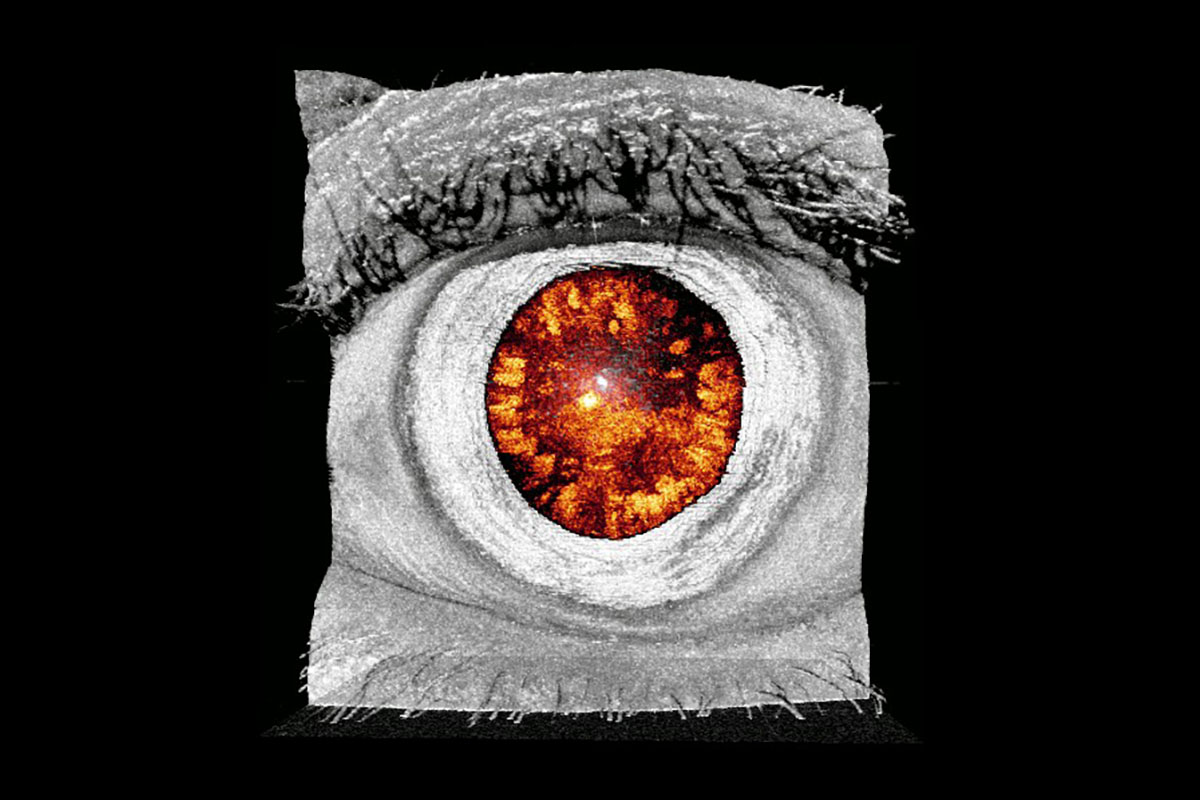
This will be complemented by the project of R2 based at IMO, who will develop new methodologies for the automated clinical evaluation of the visual function based on the precise analysis of ocular movements acquired with eye-tracking technology, to look for patterns that enable early and objective diagnosis of disorders. R3 based at UPC will extensively exploit multispectral imaging together with machine learning to develop new systems and methods for the analysis of eye structures, retinal oximetry and the automated diagnosis and monitoring of retinal diseases. The project of R4 based at NCU will focus on the development of a novel tomographic micro-vibrography system for assessing biomechanical properties of ocular structures (cornea, lens, sclera) based on the combination of Optical Coherence Tomography (OCT) and optical lock-in detection scheme for high frequency vibrometric measurements.
To advance in the clinical application of LEAP it is crucial to develop computational models of the heart, which are a powerful way to integrate physical and physiological knowledge in data analysis useful for diagnosis, prognosis and therapy planning. The project of R6 based at UPC will develop new algorithms able to provide early warning indication of dynamical transitions to critical states such as arrhythmia and cardiac defibrillation suitable for clinical use. Pulse rate, blood flow and vascularization maps are critical for diagnosis of cardiovascular diseases.
R7 based at PG will focus on advancing the state-of-the-art of multimodal imaging systems, analysing reflected light changes caused by pulsatility of blood in skin facial arteries and thermal imaging. Super-resolution and deblurring machine learning algorithms will be developed to obtain reliable estimations of vascularization maps, which are critical for diagnosis. R8 based at UZH will further develop multispectral optoacoustic tomography using image reconstruction methodologies and deep learning methods. The goal pursued is identifying carotid artery stenosis resulting from a build-up of atherosclerotic plaques, which is a major risk factor for ischemic stroke.
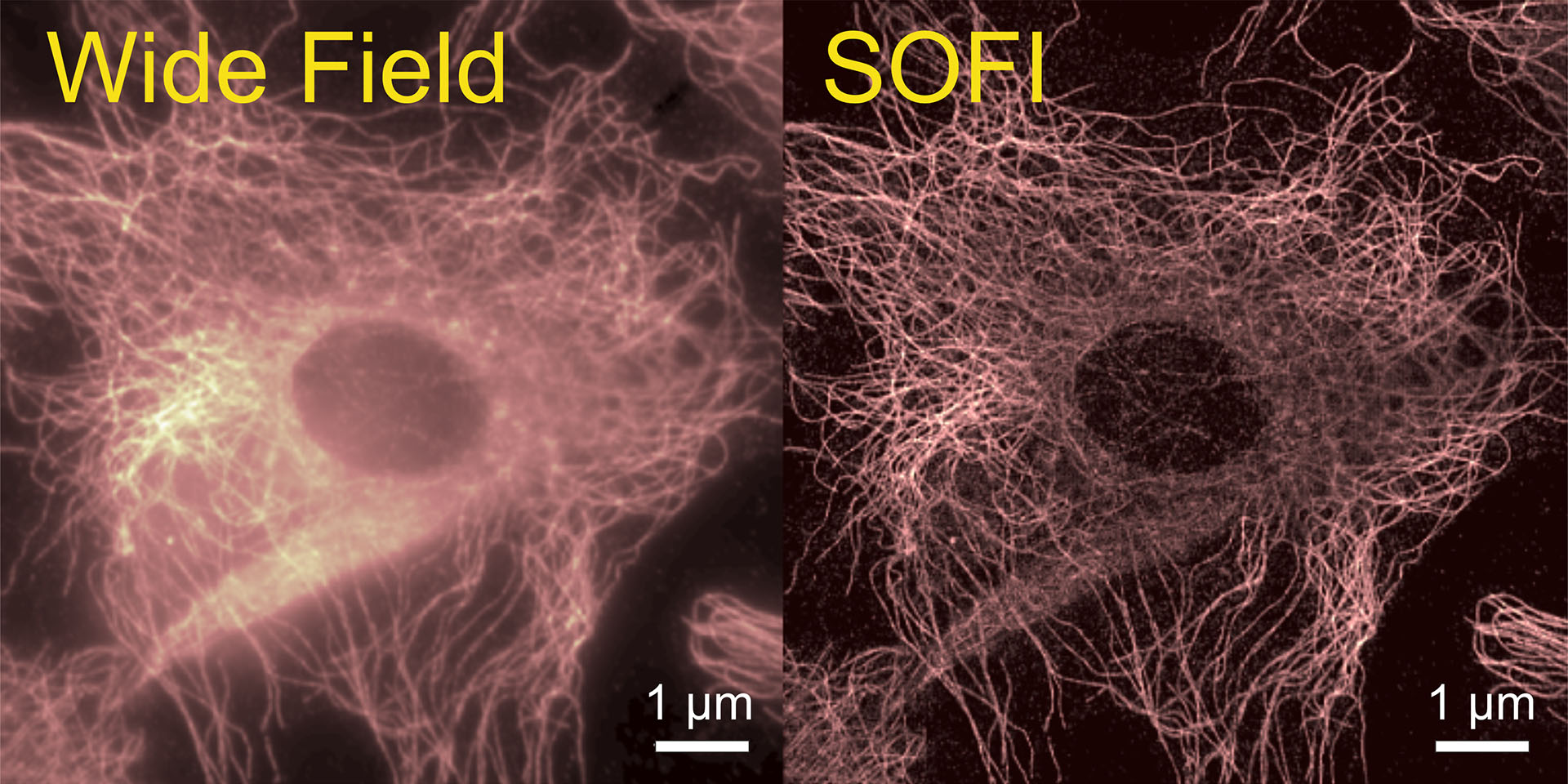
The last decades have seen a revolution in optical microscopy with the invention of many different methods to increase the spatial resolution beyond the diffraction limit, allowing for resolving structural details in cells and tissues. One of them is Super-resolution Optical Fluctuation Imaging (SOFI), which exploits stochastic intensity fluctuations of emitters and uses a mathematical cumulant analysis for converting these temporal fluctuations into an increased spatial resolution. SOFI performance can be dramatically improved by applying new statistical data analysis tools from machine and deep learning. The goal of the project of R9 based at UGOE is to use new approaches that can advance the performance of SOFI in terms of speed, spatial resolution, and 3D sample reconstruction.
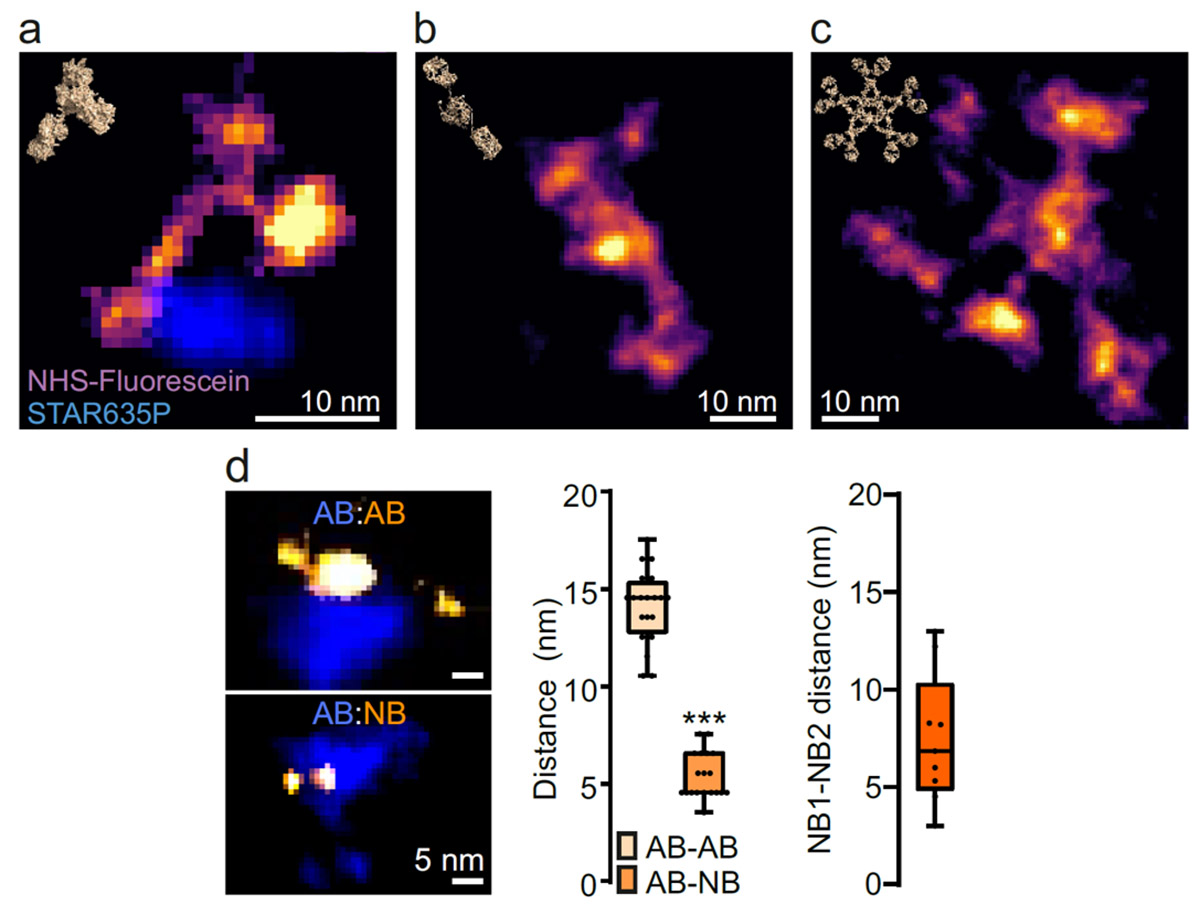
Complementing these efforts, the project of R10 based at UMG will apply One-step Nanoscale Expansion (ONE) microscopy (which has been shown to achieve spatial resolutions down to 1 nm) and machine learning for the automated analysis of the shape of single molecules and molecular complexes, which represents a promising new diagnosis, especially for neurodegenerative conditions such as Parkinson’s disease. Finally, R11 based at FSJD will provide novel methods based on super-resolution microscopy (STimulated Emission Depletion microscopy-STED, and computational super-resolution) and deep learning that can aid diagnosis of genetic and acquired rare diseases (e.g., muscular and neurogenetic dystrophies).